Aluminum Sulfide Reacts With Water. What mass of the excess reactant remains? Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide.
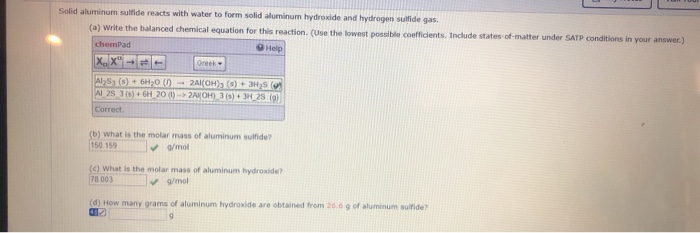
Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide. (b) how many grams of aluminum hydroxide are obtained from 14.2 g of aluminum sulfide? In water purification, it causes suspended impurities to coagulate into larger particles and then settle to the bottom of the container (or be filtered out) more easily.
Write The Balanced Chemical Equation For This Reaction.
How many grams of aluminum hydroxide are obtained from 16.2 of aluminum sulfide? Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide. Write the balanced chemical equation, and determine the number of grams of aluminum hydroxide that are obtained from 14.2g of aluminum sulfide?
Learn This Topic By Watching Limiting Reagent Concept Videos Frequently Asked Questions
Express your answer as a balanced chemical equation. Aluminum sulfide react with water al 2 s 3 + 6h 2 o → 2al (oh) 3 + 3h 2 s [ check the balance ] aluminum sulfide react with water to produce aluminium hydroxide and hydrogen sulfide. Hydrogen gas and aluminum chloride solution are produced when solid aluminum is reacted with hydrochloric acld.
Aluminum Sulfide Reacts With Water To Form Aluminum Hydroxide And Hydrogen Sulfide A.
Click here👆to get an answer to your question ️ problem #5: Receive better content over the first ionization energy 𝐸 of which water reacts with to aluminum water form aluminum sulfate compounds may work. If 10.6 grams of aluminum hydroxide is produ
Askedjun 30, 2017In Chemistryby Beenx A) 0.12 Moles B) 0.50 Moles C) 0.78 Moles D) 2.0 Moles
Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide. Paulesparsa6 show answer this type of reaction is a metathesis. Aluminum and its reaction with water what makes aluminum corrosion resistant.
Aluminum Sulfide Reacts With Water To Form Aluminum Hydroxide And Hydrogen Sulfide.
He conducted his experiment with. Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide by the following reaction al2s3 + 6 h2o → 2al(oh)3 + 3h2s if 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed? The gravimetric hydrogen capacity from this reaction is 37 wt and the volumetric hydrogen capacity is 46 g h.
Related Posts
- What Evidence Do Paleobotanists Look For That Indicates The Movement Of Plants From Water To LandWhat Evidence Do Paleobotanists Look For That Indicates The Movement Of Plants From Water To Land. A) waxy cuticle to decrease evaporation from leave ...
- Adjectives That Start With F To Describe A Person PositivelyAdjectives That Start With F To Describe A Person Positively. Adjectives starting with g!all the adjectives that start with g to describe a person po ...
- How Many Ounces Is In A Nestle Water BottleHow Many Ounces Is In A Nestle Water Bottle. A typical water bottle holds 500 ml of water. The bottle is roughly 8″ tall with a circumference where t ...
- How Do You Divide Exponents With Different BasesHow Do You Divide Exponents With Different Bases. In order to divide exponents with different bases and the same exponent, we use the 'power of ...
- States That Start With MStates That Start With M. There are 8 states that start with:montanaminnesotamichiganmissourimarylandmassachusettsmainemississippi. Capital largest ( ...
- Fahrenheit 451 Important Quotes With Page NumbersFahrenheit 451 Important Quotes With Page Numbers. Fahrenheit 451 quotes | explanations with page numbers | litcharts. 'fahrenheit 451' quo ...
- Assign Currentsize With The Size Of The Sensorreadings VectorAssign Currentsize With The Size Of The Sensorreadings Vector. Int main() { vector sensorreadings(4); May anyone help guide and explain this program ...

