Bond Angle Of Bf3. Why bond angle in nf3 less than that in nh3? Bi3 > bbr3 > bcl3 > bf3.
So, the correct order of bond angle will be bbr 3 > bcl 3 > bf 3. Select the correct value for the indicated bond angle in each of the following compounds: Does the bond length increase with the bond angle in other cases of such kinds of molecules?
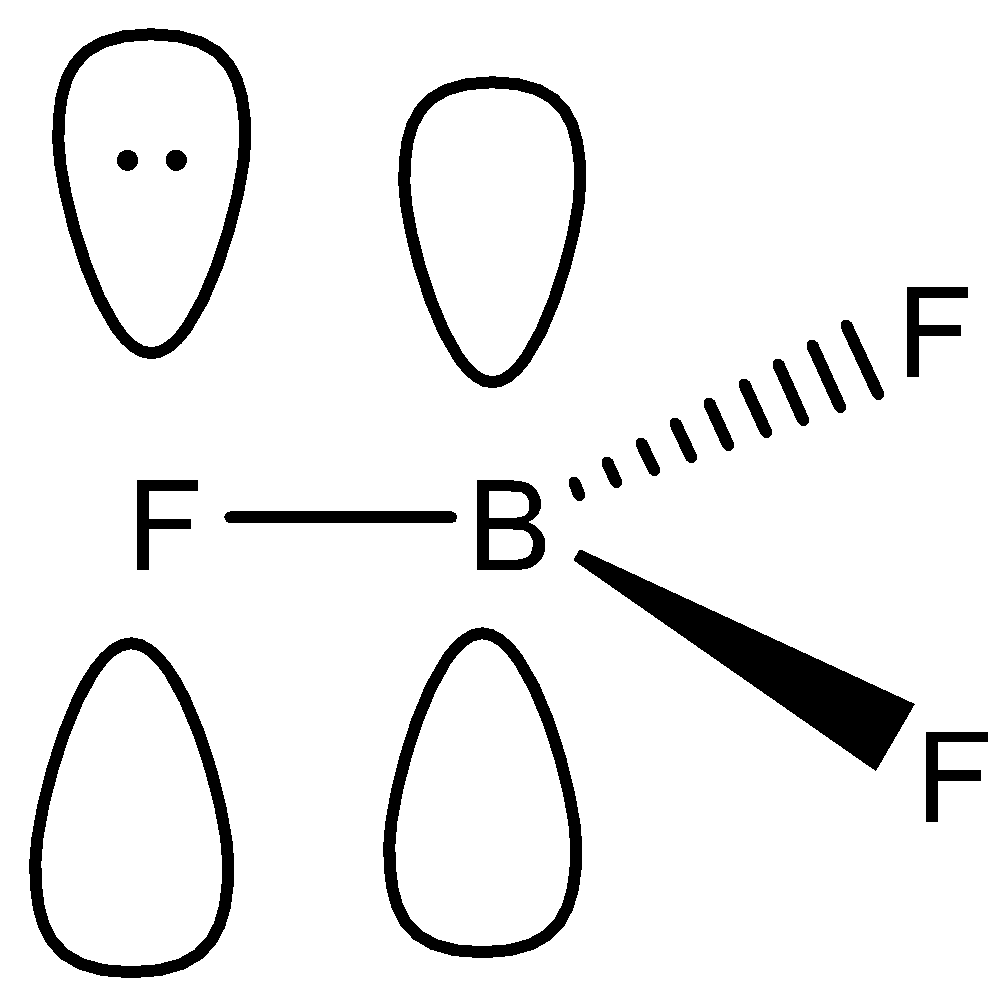
The Geometry Of The Bf 3 Molecule Is Called Trigonal Planar (See Figure 5).
The geometry of molecule of bf3 is ‘trigonal planar.’ with the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. So, in the case of nf3, fluorine is more electronegative than nitrogen hence it attracts more electrons. The order of electronegativity of halides is f> cl > br.
So, The Correct Order Of Bond Angle Will Be Bbr 3 > Bcl 3 > Bf 3.
A fluorine atom is positioned at 120°, which is bonded with the center atom boron in the lewis structure. The atomic size of the halogen is directly proportional to the bond angle. Therefore, the resultant net dipole moment of the bf3 molecule is zero which means the boron trifluoride (bf3) has no dipole moment (μ=0d).
Figure Out The Vsepr Shape.it's Trigonal Planar3.
Select the correct value for the indicated bond angle in each of the following compounds: Click on to see full reply The bond angle is 1 2 0 o.
Answered By Expert 4Th December 2017, 10:31 Am.
A) b f 3 has trigonal planar geometry with 3 bond pairs. In bf3, bond angle is : The bond angle in bf3 is 120 degrees, but in ncl3 is 103 degrees.
Bcz The Structure Of Bf3 Is Trigonal Planar With Sp2 Hybridization So Bond Angle Is 120The Structure Of Nh3 Is Trigonal Pyramid With A Lone Pair The Hybridization Is.
To be more precise, the bf 3 molecular geometry is trigonal planar. According to the vsepr theory, bf3 possesses trigonal planar molecular geometry. The atomic size of the halides plays a vital role for bond angle in boron halides.
Related Posts
- The Angle Addition Postulate AnswersThe Angle Addition Postulate Answers. Post the pages around your room in a random order. Segment addition postulate worksheet answers free segment pr ...
- Is Hbr A Polar Covalent BondIs Hbr A Polar Covalent Bond. What causes a bond to be polar? Now, hbr hasone single bondand six electrons or three lone pairs around bromine.Covalen ...
- Define Vertex Angle Of An Isosceles TriangleDefine Vertex Angle Of An Isosceles Triangle. The vertex opposite the base is called the apex. It has an axis of symmetry along its vertex heightSolv ...
- Sef4 Bond AnglesSef4 Bond Angles. What is the molecular geometry of sef4? Sef4 bond polarity and dipole moment the polarity of a molecule can be determined by checki ...
- Cs2 Bond AngleCs2 Bond Angle. Polarity in dichlorine monoxide (ocl2) Hybridization of the given molecule h2s is sp3;PPT VSEPR THEORY (Valence Shell Electron Pair R ...
- The Vertex Angle Of An Isosceles Triangle Measures 40° What Is The Measure Of A Base AngleThe Vertex Angle Of An Isosceles Triangle Measures 40° What Is The Measure Of A Base Angle. What is the measure of a base angle? If the vertex angle ...
- Pf5 Bond AnglePf5 Bond Angle. The best predicted shape and bond angle of sbh 3 is 1. Determine the shape and ideal bond angle(s) of pf5.PF5 Lewis structure, Molecu ...
