Lewis Structure Of Cos. The lewis structure (lewis dot diagram) for co.1. Hybridization is the process of mixing two or more atomic orbitals to create new covalently bonded orbitals in molecules.
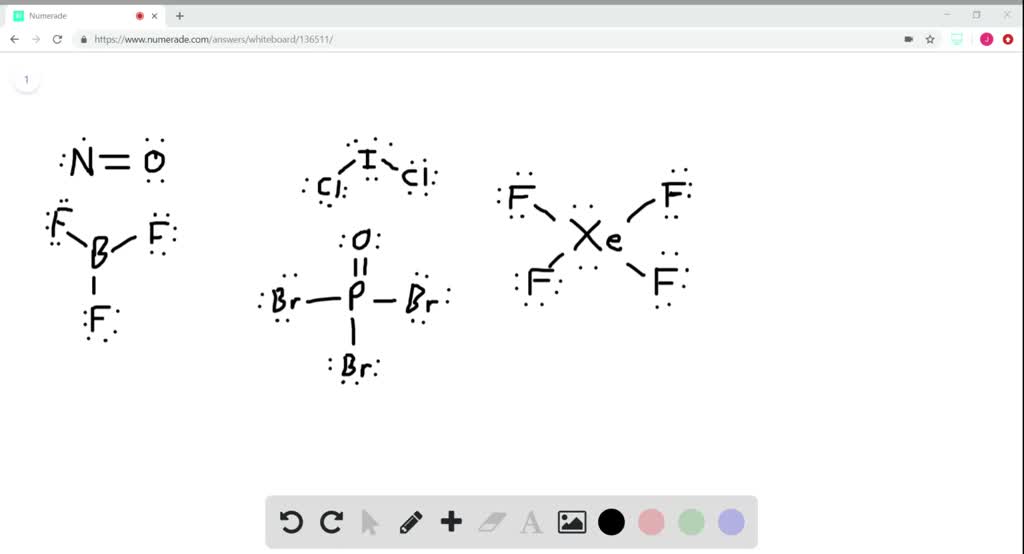
Discovered by chevrenl in 1913, cetyl alcohol is one of. However, eventhough linear geometries normally represent nonpolar lewisstructures, o is much. Bromine is the third member of the halogen family.
We Have 16 Valence Electrons For The Ocs Lewis Structure.
It can be produced from the reduction of palmitic acid.cetyl alcohol is present in a waxy white powder or flake form at room temperature, and is insoluble in water and soluble in alcohols and oils. Discovered by chevrenl in 1913, cetyl alcohol is one of. In addition to this, the contribution of the lone pair of electrons further contributes to the linear geometry of.
The Lewis Structure For Cl 2 Co Requires You To Place Carbon In The Center Of The Structure Since It Is The Most Electronegative.
Carbon is less electronegative than oxygen, so it won't be happy with having more electrons than oxygen. The best lewis structure for sulfuric acid has zero formal charges, sulfur as the central atom, and no bonds between s and h. For carbonyl sulfide we have 4 +6 + 6 valence electrons, for a total of 16 valence electrons for the cos molecule.
Lewis Structure Of Ch2Br2 For Constructing Around The More Electronegative Atom.
Bromine is the third member of the halogen family. Build your own widget » browse widget gallery » learn more » report. Put one electron pair in each bond4.
Rules For Writing Lewis Structures.
0) cos, (i) ccla, (ii) nfs. Carbon is the least electronegative. The molecule is made up of one phosphorus atom and three bromine atoms.
All Of The Geometries Listed Below Are Examples Of The Five Basic Geometries For Molecules With More Than 3 Atoms Except A.
Count the total number of valence electrons in the molecule or polyatomic ion. Fc = 0 but carbon does not have an octet, so this is not realistic. We'll put the oxygen on one side and sulfur on the other.
Related Posts
- Icl Lewis StructureIcl Lewis Structure. For icl2+, the central i has 2 lone pairs and 2 shared pairs. Draw the lewis structure for the molecule.ICl4^의 루이스 구조. Lewis str ...
- Bro3 Lewis StructureBro3 Lewis Structure. All of the bonds in the bromate ion are identical. Joey mentioned two double bonds to two of the oxygen.BrO3 Lewis Structure Ho ...
- Ch4 Electron Dot StructureCh4 Electron Dot Structure. Calculate the total valence electrons in the molecule. Lewis dot structure of ch 4.Ch4 Lewis Dot Diagram lewis dot struct ...
- Lewis Structure BrfLewis Structure Brf. Lone pairs take part in hybridization. Note that in the lewis structure for brf5, bromine (b) is in period four on the.Bromine p ...
- Chlorine Lewis Dot StructureChlorine Lewis Dot Structure. Both the atoms are the same. Chlorine is the second lightest halogen and is represented as cl.Media Portfolio from wps. ...
- Xef2O Lewis Structure Formal Charge 0Xef2O Lewis Structure Formal Charge 0. There are a total of 22 valence electrons in the lewis structure for xef2. See the answer see the answer see t ...
- Lewis Dot Structure For Ch3Ch2OhLewis Dot Structure For Ch3Ch2Oh. Two carbon atoms have joint with a single bond and oxygen atom has made bonds with carbon and hydrogen atoms. Ethan ...




