Co32 Valence Electrons. Therefore it is put in the center of the dot structure. Carbonate has a central carbon atom bonded to three oxygen atoms.
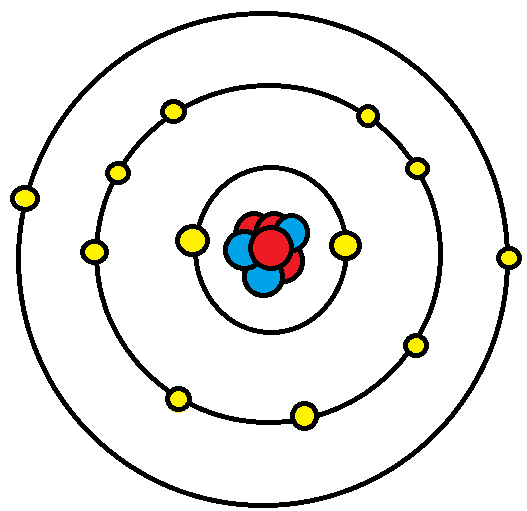
Oxygen has six, we have 3 oxygens, and this negative 2 means we have an extra two valence electrons. Considering this, what is the hybridization of h2o? Carbon has 4 valence electrons;
Total Electron Pairs Are Determined By Dividing The Number Total Valence Electrons By Two.
So, carbon has four electrons in its valence shell.oxygen is located at 6 th group. Just add up the valence electrons in each atom and then adjust to match the charge if any. Two electrons have been shared between carbon and each of the three oxygen atoms which point towards the existence of single bonds.
Now, Clearly, The Ion Must Contain 2 More Electrons, Than It Does Nucular Charges.in One Formula Unit Of Co2− 3 There Are 6C +3 ×8O = 30 ⋅ Positively Charged Nuclear Particles.
Then, what is the hybridization of co 32? For determining the lewis structure for any molecule, we first need to know the total number of valence electrons.these electrons are the ones that are present in the outermost shell of the atom and participate in forming bonds. H2o has 2 (1) + 6 = 8 valence electrons.
Which Of The Following Statements Is True?
Carbonate has a central carbon atom bonded to three oxygen atoms. Valence electrons of o= 6 o = 6. Level 1 · 2 days ago.
The Carbonate (Co2−3) Ion Carbon Has 4 Valence Electrons, Each Oxygen Has 6 Valence Electrons, And There Are 2 More For The −2 Charge.
The total number of valence electrons has been made equal to 24. How do you find the formal charge? Draw the lewis structure of the carbonate ion, co 32−.
If There Were Three Single Bonds To Oxygen And A Lone Pair On Carbon Then The Molecule Would Have 4 Negative Charges.
C => 4 o => 6 (but there are two oxygens) =>12 4+12 = 16. Valence electrons of c= 4 c = 4. C= 4 x 1 = 4.
Related Posts
- Valence Electrons MagnesiumValence Electrons Magnesium. From both the electronic configurations it is clear that magnesium has two valence electrons whereas oxygen has six vale ...
- Number Of Valence Electrons In FluorineNumber Of Valence Electrons In Fluorine. The last shell after the electron configuration is called the valence shell. Thus,fluorine has seven valence ...
- Number Of Valence Electrons In IronNumber Of Valence Electrons In Iron. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we& ...
- Valence Electrons In MgValence Electrons In Mg. The only way for magnesium to accomplish this is to give up its two valence electrons and have one of its filled energy leve ...
- Valence Electron For IronValence Electron For Iron. Iron 26 has 26 protons, 29 neutrons, and 26 electrons. There are two types of iron ions.iron Element, Occurrence, Uses, Pr ...
- Valence Electrons Of StrontiumValence Electrons Of Strontium. In the periodic table, the elements are listed in order of increasing atomic number z. Possible oxidation states are ...
- How Many Valence Electrons Does Tl HaveHow Many Valence Electrons Does Tl Have. Thallium is also present in manganese nodules found on the ocean floor. How many valence electrons does lead ...





