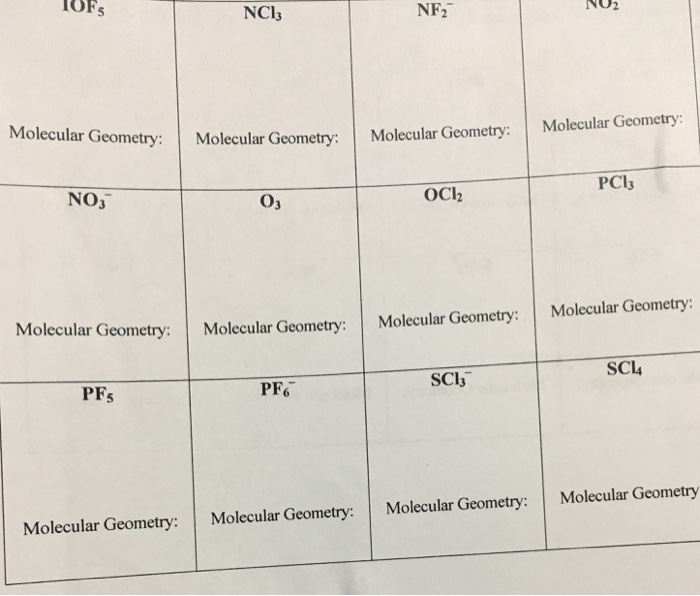
Ncl3 Geometry. Electron geometry for this is tetrahedral. A) eg=tetrahedral, mg=tetrahedral b) eg=linear, mg=trigonal planar c) eg=trigonal planar, mg=bent d) eg=linear, mg=linear e) eg=tetrahedral, mg=trigonal pyramidal
Symmetrical geometric shape and symmetrical distribution of charge! 2 or more lines of symmetry when you cut through the middle of the molecule; A quick explanation of the molecular geometry of ncl3 (nitrogen trichloride) including a description of the ncl3 bond angles.looking at the ncl3 lewis struct.
Symmetrical Geometric Shape And Symmetrical Distribution Of Charge!
Molecular geometry of nitrogen trichloride (ncl3) nitrogen trichloride is a tetratomic molecule as it has three chlorine atoms bonded with a single nitrogen atom having one lone pair of valence electrons. Determine the electron geometry (eg) and molecular geometry (mg) of ncl3. It can be observed from the lewis structure that iodine, the central atom, has three bond pairs and two lone pairs of electrons.
It Makes The Molecular Geometry Of Nitrogen Trichloride Trigonal Pyramidal.
C) eg = trigonal planar, mg = bent. Eg = linear, mg = trigonal planar There are two lonepairs around oxygen, which make up the last two electron groups.
Molecules With Four Electron Groups Has A Tetrahedral.
The three atoms of chlorine bonds with the nitrogen atom leaving behind a lone pair on the nitrogen atom. It makes the molecular geometry of. The three atoms of chlorine bond with the nitrogen atom (by a single bond), i.e.
Determine The Electron Geometry (Eg) And Molecular Geometry (Mg) Of Ncl 3.
Nitrogen and chlorine are both nonmetals and bonds between nonmetals tend to be covalent. The molecular geometry of nbr3 is trigonal pyramidal, and electron geometry is tetrahedral because the lone pair present on the central atom creates repulsion between adjacent bonded pairs of electrons, as a result, two bromine atom in equatorial position pushes far apart giving its molecular geometry same as a trigonal pyramid. Ncl3 is a slightly polar molecule.
Determine The Electron Geometry (Eg) And Molecular Geometry (Mg) Of Ncl3.A.
Electron geometry for this is tetrahedral. D) eg = linear, mg = linear. Eg = tetrahedral, mg = trigonal pyramidalb.
Related Posts
- Electron Geometry Cocl2Electron Geometry Cocl2. What is the shape (molecular geometry) of cocl 2? Cocl2 molecule consists of one c, one o, and cl atoms.The Shapes of Molecu ...
- So42 Molecular GeometrySo42 Molecular Geometry. What type of bond is so42? Molecular geometry & polarity tutorial.Is SO42 Polar or Nonpolar? (sulfate ion) YouTube from ...
- H2S Electron GeometryH2S Electron Geometry. The total valence electron in h2s is 8. Hno2 molecular geometry the next important step is to determine the molecular geometry ...
- Molecular Geometry Of Cocl2Molecular Geometry Of Cocl2. Pf3 has 26 valence electrons. 8 rows the chemical formula cocl2 represents cobalt (ii) chloride.Fosgene from www.lookfor ...
- Determine The Molecular Geometry Of Cbr4Determine The Molecular Geometry Of Cbr4. To carry out so, we an initial need to draw alewis framework for cbr4.because that this, we need to do the ...
- Tecl4 Electron Domain GeometryTecl4 Electron Domain Geometry. Find the electron domain geometry & the molecular geometry of the following compounds: Furthermore, what is the e ...
- Xeo2F2 GeometryXeo2F2 Geometry. Total valence shell electrons pairs: Xeo2f2 is polar and it helps to identify this through drawing out the lewis structure.Solved 9. ...



