Xeo2F2 Geometry. Total valence shell electrons pairs: Xeo2f2 is polar and it helps to identify this through drawing out the lewis structure.
On partial hydrolysis, xef6 gives: Both fluorine and oxygen are highly electronegative and can cause electron shift from f. (b) the hybridization of xeo3f2 is sp3d and its structure is trigonal bipyramidal in which oxygen atoms are situated on the plane and the fluoride atoms are on the top and bottom.
Of Electrons In Outer Most Shell
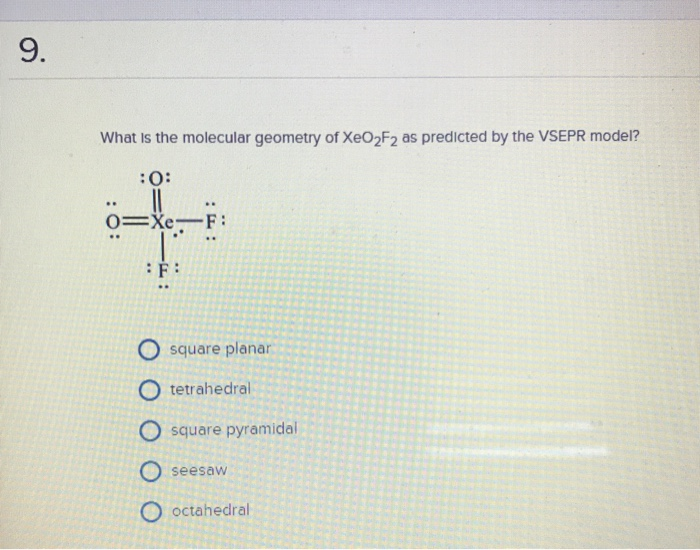
It is a type of noble gas having the chemical equation of. ← prev question next question. The shape of xeo2f2 molecule is (a) trigonal bipyramidal (b) square planar (c).
What Is The Electron Geometry Of Of2?
It supports sp 3 d 2 hybridisation. Xenon oxytetrafluoride (xe o f 4) is an inorganic chemical compound.it is a colorless stable liquid with a melting point of −46.2 °c that can be synthesized by partial hydrolysis of xef 6, or the reaction of xef 6 with silica or nano 3:. We have already found the 2d lewis structure diagram of the oxygen difluoride molecule.
Electron Geometry For This Is Tetrahedral.
The molecular symmetry and crystal structure of xeo2f2 have been determined from single crystal neutron diffraction data. What is the molecular geometry of xeo2f2 as predicted by the vsepr theory? The central atom in xeo2f2 is xeit belongs to viii a group in the periodic table and has 8 valence e around this xe atoms there are two oxygen divalent atom atoms two fluorine mono vale view the full answer.
The Xef4 Or Xenon Tetrafluoride Is A Chemical Compound Made Of Xenon And Fluoride Atoms.
Hence the hybridization will be sp3d. Nano 3 + xef 6 → naf + xeof 4 + fno 2. It is the world’s first binary compound discovered.
(A) It Can Reduce Tollen’s Reagent However Cannot Reduce Fehling’s Reagent (B) It.
The xef4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form. The shape is linear because the lone pairs prefer the equatorial positions. Veamos como ejemplo la primera molécula, pcl3, localiza al fósforo (el átomo central, lo sabes porque es el menos electro negativo) en la tabla periódica, nota que pertenece al grupo va, o sea que tiene 5 electrones en la capa e valencia,.
Related Posts
- Determine The Molecular Geometry Of Cbr4Determine The Molecular Geometry Of Cbr4. To carry out so, we an initial need to draw alewis framework for cbr4.because that this, we need to do the ...
- Molecular Geometry Of Cocl2Molecular Geometry Of Cocl2. Pf3 has 26 valence electrons. 8 rows the chemical formula cocl2 represents cobalt (ii) chloride.Fosgene from www.lookfor ...
- So42 Molecular GeometrySo42 Molecular Geometry. What type of bond is so42? Molecular geometry & polarity tutorial.Is SO42 Polar or Nonpolar? (sulfate ion) YouTube from ...
- H2S Electron GeometryH2S Electron Geometry. The total valence electron in h2s is 8. Hno2 molecular geometry the next important step is to determine the molecular geometry ...
- Tecl4 Electron Domain GeometryTecl4 Electron Domain Geometry. Find the electron domain geometry & the molecular geometry of the following compounds: Furthermore, what is the e ...
- Ncl3 GeometryNcl3 Geometry. Electron geometry for this is tetrahedral. A) eg=tetrahedral, mg=tetrahedral b) eg=linear, mg=trigonal planar c) eg=trigonal planar, m ...
- Electron Geometry Cocl2Electron Geometry Cocl2. What is the shape (molecular geometry) of cocl 2? Cocl2 molecule consists of one c, one o, and cl atoms.The Shapes of Molecu ...



