Difference Between Molecular Compound And Ionic Compound. Composition varies depending on the type of compound, but all ionic compounds must have a neutral electric charge. Compounds are pure substances that are combinations of two or more elements.
The ionic compounds will generally be high melting brittle solids, molecular compounds will vary from. The electronegativity difference between oxygen and hydrogen is high which is why water has a positive pole of h and a negative pole o ( water is h2o ). Carbon monoxide (co) is an example of a diatomic compound.
Ionic Compounds Are Ionic Compounds That Are Bound Together By Ionic Bonds.
Ionic compounds are formed when ionic bonds are formed between different elements through the transfer of electrons. An ionic compound consists of two oppositely charged ions. Carbon monoxide (co) is an example of a diatomic compound.
A Molecule Is An Electrically Neutral Group Of Two Or More Atoms Joined By Chemical Bonds.
Molecular compounds are pure substances formed when atoms are linked together by sharing of electrons while ionic compounds are formed due to the transfer of electrons. Atomic numbers distinguish between distinct types of elements, while compounds are characterised by their fixed ratio of different elements in a certain arrangement. The difference between ionic and molecular compounds is that the electrons of atoms in ionic compounds are transferred between the elements because of the presence of a difference in electronegativity.
Ionic Compounds Are Binary Compounds That Come Under Two Different Categories.
Molecular compounds are made due to covalent bonding while ionic. Main differences between ionic vs molecular compounds. A molecule is a group of two or more atoms held together by chemical bonds.
Thus They Have High Melting And Boiling Points.
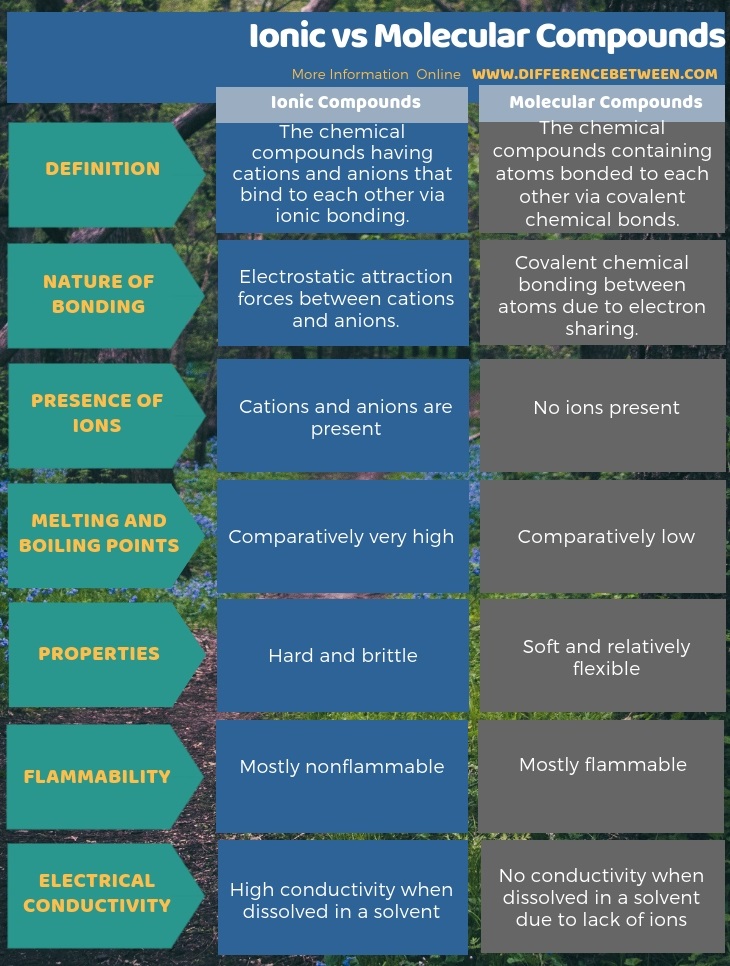
Differences between ionic and molecular compounds. Covalent bonds connect molecules together. In showing the difference between molecular and ionic compounds, here is a tabular representation of their most important variations.
The Main Difference Between Both Compounds Is That Ionic Compounds Are Formed By The Transfer Of Electrons While In Molecular Compounds Electron Is Shared Amongst Constituent Elements.
Ionic compound is a chemical compound in which the bonding between atoms is achieved through the electrostatic attraction between oppositely charged ions, while covalent compound is a chemical compound in which the atoms share electrons in order to attain the electron configuration of the outermost shell. I'm gonna have to disagree with both of you, assuming i understand what the person is really asking here. Compounds are pure substances that are combinations of two or more elements.
Related Posts
- What Is The Difference Between Structuralism And FunctionalismWhat Is The Difference Between Structuralism And Functionalism. Posted on january 27, 2022; Understanding the conscious experience through introspect ...
- Molecular Geometry Of Cocl2Molecular Geometry Of Cocl2. Pf3 has 26 valence electrons. 8 rows the chemical formula cocl2 represents cobalt (ii) chloride.Fosgene from www.lookfor ...
- H2So4 Naoh Net Ionic EquationH2So4 Naoh Net Ionic Equation. The net ionic equation (a chemical equation in which electrolytes are written as dissociated ions) can be explained as ...
- Predict Whether Each Of The Following Ionic Compounds Is Soluble In WaterPredict Whether Each Of The Following Ionic Compounds Is Soluble In Water. Generally, we can apply the like dissolves like rule to determine if a com ...
- Molecular Mass Of PbMolecular Mass Of Pb. Its atomic weight (average relative mass ) on the periodic table is 207.2. Convert grams pb (aso4)2 to moles or moles pb (aso4) ...
- Nabr Compound NameNabr Compound Name. Computed by cactvs 3.4.8.18 (pubchem release 2021.05.07) hydrogen bond acceptor count: Visit byju's to understand the proper ...
- The Gravitational Force Between Two Objects Of Masses M1 And M2 That Are Separated By Distance R IsThe Gravitational Force Between Two Objects Of Masses M1 And M2 That Are Separated By Distance R Is. What does the force of gravity between two objec ...


