H2So4 Naoh Net Ionic Equation. The net ionic equation (a chemical equation in which electrolytes are written as dissociated ions) can be explained as follows: H2so4 (aq) + mg (oh)2 (aq) →mgso4 (aq) + 2h2o (l) does the question ask for net ionic equations?
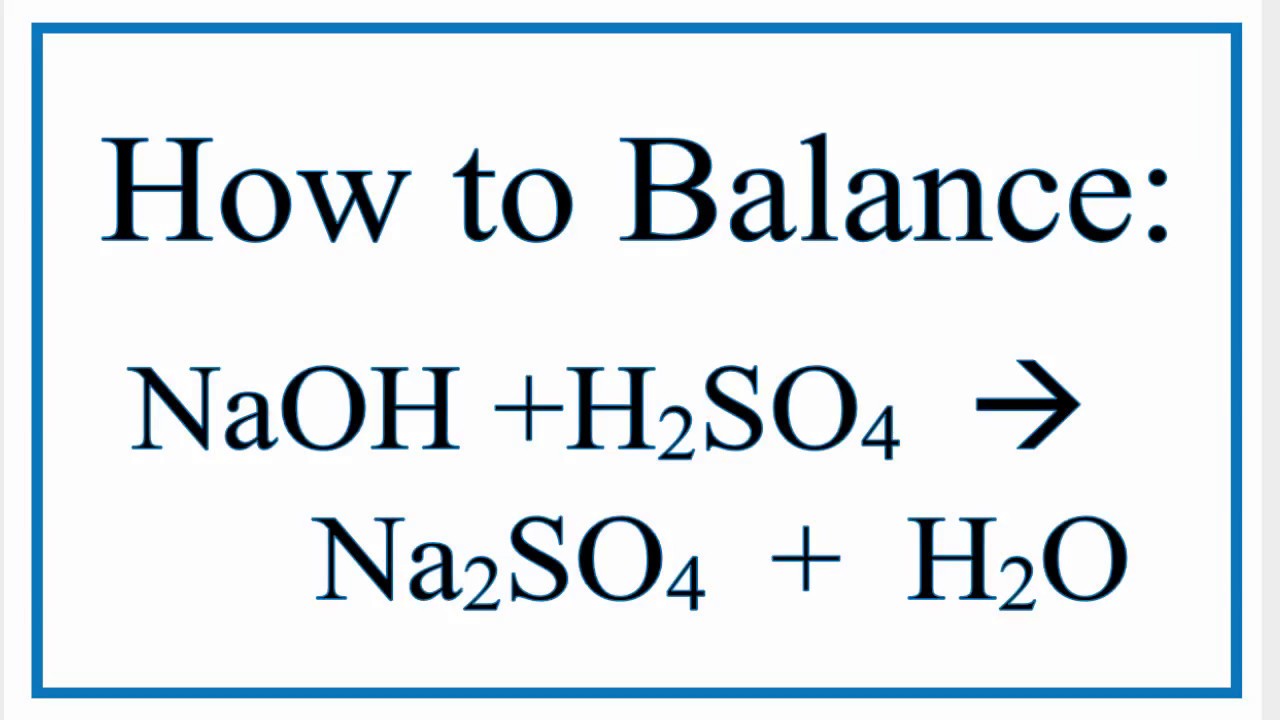
How to balance the net ionic equation for naoh + ch 3. I'm assuming that you're talking about the last reaction—h2so4 (aq) + 2 naoh (aq) → na2so4 (aq) + 2 h2o (ℓ).when we break this up into its ions, we get h2 (aq) + so4(2−) (aq) + 2 na+ (aq) + 2 oh− (aq) → 2 na+ = so4(2−) (aq) + 2. Zn(cl)2 and naoh reaction creates nacl and zn(oh)2.
H 3P O4(Aq) + 2H O− → H P O2− 4 + 2H 2O.
Add your answer and earn points. Naoh + h2so4 = na2so4 + h2o is not a redox reaction. Then, what is the net ionic equation of the reaction of zncl2 with naoh?
Equation For H2So4 And Naoh Upvote7Downvote0Shareanswer It4 Good Habit Start Inspecting The Reaction Writing The Balanced Equation Naoh H2So4 2H2O Na2So4 This Titration Problem.simply So, What Salt Produced.
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqueous sodium sulfate (na2so4) and water. H⁺ (aq) + no₃⁻ (aq) + na⁺ (aq) + oh⁻ (aq) → na⁺ (aq) + no₃⁻ (aq) + h₂o (l) 2. Ca (oh)2 > cao + h2o b.
Strong Acids And Strong Bases Are Considered Strong Electrolytes And Will Dissociate Completely.
H2so4 (aq) + mg (oh)2 (aq) →mgso4 (aq) + 2h2o (l) does the question ask for net ionic equations? 4k + o2 → 2k20 which statement is true? Zn(cl)2 and naoh reaction creates nacl and zn(oh)2.
How Do You Balance H2So4 Naoh?
H2so4 + 2naoh → na2so4 + 2h2o the ionic equation omits ions which act as spectators so we can split the reactants and the products into ions to see which are the spectators. The overall equation for this reaction is: When h2so4 is neutralized by naoh in aqueous solution, the net ionic equation is.
Thus, Writing This In A Full Ionic Reaction Form , We Get:
What happens when you mix h2so4 and naoh? Naoh (s)+ h2so4 (aq)=na2so4 (aq) + h2o (l) consider the unbalanced equation above.a 0.900 g sample of impure naoh was dissolved in water and required 37.0 ml of 0.145 m h2so4 solution to react with the naoh in the sample. H 3p o4(aq) + h o− → h 2p o− 4 +h 2o.
Related Posts
- Which Of The Following Shows The Extraneous Solution To The Logarithmic Equation BelowWhich Of The Following Shows The Extraneous Solution To The Logarithmic Equation Below. Finally, we note that because sin and 100 are both odd functi ...
- Are Ionic Or Covalent Bonds StrongerAre Ionic Or Covalent Bonds Stronger. But, when molecules with ionic bonds are dissolved in water the ionic bonds become much weaker in comparison to ...
- Net Capital Spending EquationNet Capital Spending Equation. Net working capital is a liquidity calculation that measures a company’s ability to pay off its current liabilities wi ...
- Is Ch4 Ionic Or CovalentIs Ch4 Ionic Or Covalent. Why is water covalent, not ionic? The bonds within the compound ch4 are covalent bonds.· Formation of Carbon tetra chloride ...
- Conjugate Base For H2So4Conjugate Base For H2So4. The way we would be able to identify a conjugate base is if it has only one proton was removed from it in the acidic form. ...
- For The Oxidation Reduction Reaction Equation Given Here 2K F2For The Oxidation Reduction Reaction Equation Given Here 2K F2. Learn this topic by watching balancing redox. Oxidation reaction entails loss of elec ...
- Difference Between Molecular Compound And Ionic CompoundDifference Between Molecular Compound And Ionic Compound. Composition varies depending on the type of compound, but all ionic compounds must have a n ...

