Are Ionic Or Covalent Bonds Stronger. But, when molecules with ionic bonds are dissolved in water the ionic bonds become much weaker in comparison to covalent bonds after molecules with covalent bonds have been dissolved in water. Covalent bonds are stronger than ionic bonds.
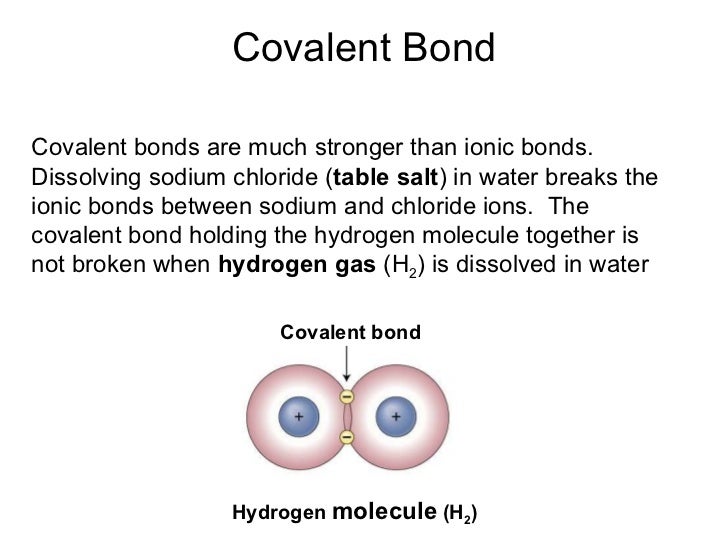
There are a number of things to consider when thinking about this:1. But, when molecules with ionic bonds are dissolved in water the ionic bonds become much weaker in comparison to covalent bonds after molecules with covalent bonds have been dissolved in water. Ionic bonds are usually stronger than covalent bonds because there is an attraction between oppositely charged ions.
But, When Molecules With Ionic Bonds Are Dissolved In Water The Ionic Bonds Become Much Weaker In Comparison To Covalent Bonds After Molecules With Covalent Bonds Have Been Dissolved In Water.
Two of the strongest forms of chemical bond are the ionic and the covalent bonds. Covalent bonds are stronger than ionic bonds. Covalent bonds are also strong due to the attraction from each nuclei to the valence electrons.2.
Covalent Bonds Hold All Of Your Biomolecules Together.
Covalent is stronger because the 2 atoms involve share 2 or more outer shell electrons. Ionic bonds are stronger than covalent bonds, because there is a stronger attraction between ions that have opposite charges, which is why it takes a lot of energy to separate them. Collected from the entire web and summarized to include only the most important parts of it.
Ionic Bonds Are Usually Stronger Bonds, As They Are Based On Electrostatic Attraction Between The Two Atoms.
Some bonds are weaker, and some are stronger. To maximize the attraction between those ions, ionic compounds form crystal lattices of alternating cations and anions. It's the electrical charge that makes them attracted to each other.
Covalent Bonding Is Dominant In Organic Chemistry, But Ionic Bonds Generally Have Higher Dissociation Energies.
This is either because the covalent bond is weak (poor orbital overlap / mismatched orbital energies) or the ionisation energies are relatively small compared to the lattice enthalpy. Ionic bonds are stronger than covalent bonds, because there is a stronger attraction between ions that have opposite charges, which is why it takes a lot of energy to separate them. You could just as well use freon (cf 2 cl 2) as an example:
Covalent Bonds Hold All Of Your Biomolecules Together.
Ionic bonds are forces that hold together electrostatic forces of attractions between oppositely charged ions. Ionic bonds are generally strong due to the attraction of oppositely charged ions. Most often ionic bonds are stronger than covalent bonds;
Related Posts
- Are Peptide Bonds CovalentAre Peptide Bonds Covalent. Similarities and differences between these two bonds are pointed out in this article. The bond that holds together the tw ...
- Is Hbr A Polar Covalent BondIs Hbr A Polar Covalent Bond. What causes a bond to be polar? Now, hbr hasone single bondand six electrons or three lone pairs around bromine.Covalen ...
- What Is The Name Of The Covalent Compound So3What Is The Name Of The Covalent Compound So3. What is the name of this compound pcl3? What covalent compound is co?PPT Nomenclature PowerPoint Prese ...
- H2So4 Naoh Net Ionic EquationH2So4 Naoh Net Ionic Equation. The net ionic equation (a chemical equation in which electrolytes are written as dissociated ions) can be explained as ...
- Difference Between Ionic Compound And Molecular CompoundDifference Between Ionic Compound And Molecular Compound. The main difference between both compounds is that ionic compounds are formed by the transf ...
- Predict Whether Each Of The Following Ionic Compounds Is Soluble In WaterPredict Whether Each Of The Following Ionic Compounds Is Soluble In Water. Generally, we can apply the like dissolves like rule to determine if a com ...
- Difference Between Molecular Compound And Ionic CompoundDifference Between Molecular Compound And Ionic Compound. Composition varies depending on the type of compound, but all ionic compounds must have a n ...


